UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
| |
||
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading symbol(s) |
Name of each exchange on which registered | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure |
Akero Therapeutics, Inc. (the “Company”) from time to time presents and/or distributes to the investment community at various industry and other conferences slide presentations to provide updates and summaries of its business. A copy of its current corporate slide presentation is being furnished herewith as Exhibit 99.1 to this Current Report on Form 8-K. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.1.
The information under this Item 7.01, including Exhibit 99.1 attached hereto is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933 or the Exchange Act, except as expressly set forth by specific reference in such filing.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit |
Description | |
| 99.1 | Corporate slide presentation of Akero Therapeutics, Inc., furnished herewith | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document). | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: June 8, 2022 | AKERO THERAPEUTICS, INC. | |||||
| By: | /s/ Andrew Cheng | |||||
| Name: Title: |
Andrew Cheng, M.D., Ph.D. President and Chief Executive Officer | |||||

Exhibit 99.1 Restoring Balance. Renewing Life. Corporate Presentation June 2022

Safe Harbor This presentation may contain “forward-looking statements” of Akero Therapeutics, Inc. (“we,” “us,” “our,” “Akero” or the “Company”) within the meaning of the Private Securities Litigation Reform Act of 1995 relating to our business, operations, and financial conditions, including but not limited to current beliefs, expectations and assumptions regarding: the future of our business; future plans and strategies, including our expectations around the therapeutic potential and clinical benefits of Efruxifermin (“EFX”); our development plans for EFX, including our belief in the unique potential of EFX as a foundational NASH therapy; our preclinical and clinical results, including our safety/tolerability, laboratory measures and paired biopsy data from our Phase 2a BALANCED study; the potential benefits resulting from the PRIME and Fast Track designations of EFX; the Phase 2b HARMONY and SYMMETRY studies, including expected timing to complete enrollment, report preliminary results, and other related milestones; the availability of a new drug product formulation to support Phase 3 clinical trials; risks related to the competitive landscape; expectations regarding the Company’s use of capital, expenses and other future financial results; and the potential impact of COVID-19 on strategy, our employees, supply chain, future operations and clinical trials. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in our most recent annual report on Form 10-K filed with the Securities and Exchange Commission, as well as discussions of potential risks, uncertainties, and other important factors in our other subsequent filings with the Securities and Exchange Commission. All information in this presentation is as of the date hereof, and we undertake no duty to update this information unless required by law. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source. ©2022 AKERO THERAPEUTICS. 2

Corporate Highlights 1 2 3 Potential First-in-Class Building Momentum Toward Experienced Team with & Best-in-Class NASH Drug Phase 3 Pivotal Trials Strong Cash Position • Substantial potential market • Two parallel Phase 2b trials underway • Involved in 20+ FDA approvals opportunity • HARMONY (F2-F3) • ~$165M cash on hand as of 1Q’22 • SYMMETRY (F4, compensated) • Differentiated mechanism of action • Cash runway into 3Q’23 • Regulatory designations • Strongest reported efficacy data • Fast Track (US FDA) among FGF21s • PRIME (European EMA) • Commercial drug product-device for Phase 3 HARMONY results expected 3Q’22 ©2022 AKERO THERAPEUTICS. 3

Extensive Development and Commercialization Experience Involved in 20+ Medicine Approvals Andrew Cheng, MD, PhD | President & CEO • 19 years at Gilead • Chief Medical Officer & HIV Division Head • Major role in 11 NDA/MAA approvals Tim Rolph, D.Phil | Co-Founder & Chief Scientific Officer Kitty Yale | Chief Development Officer • Over 25 years at Gilead, Roche, Pfizer • Over 30 years at Pfizer & Glaxo • VP, Gilead Worldwide Clinical Operations • CSO of Pfizer’s cardiovascular and metabolic disease unit • Major role in 8 global approvals NDA, MAA, JNDA • Head of Groton & UK Discovery Research, Pfizer and CFDA • Major role in discovery and early clinical evaluation of two medicines: Selzentry (HIV) and Steglatro (Diabetes) Jonathan Young, PhD, JD | Co-Founder & COO William White | CFO & Head of Corporate Development • Over 15 years in biotechnology product development, • 18 years in life sciences investment banking at Goldman law and regulatory policy Sachs, Citigroup and Deutsche Bank • General Counsel and VP Policy, Braeburn • Most recently, Head of US Life Sciences Investment Banking at Deutsche Bank • Partner and General Counsel, FoxKiser • Advised on more than $70bn in M&A and $25bn in financing transactions ©2022 AKERO THERAPEUTICS. 4

Providing a Potentially Effective Treatment for NASH Reducing liver fat Achieving >10% weight is critical to remove loss is challenging for disease driver patients who are obese Peripheral fat Insulin resistance and is the largest source of Type 2 Diabetes drives liver fat in patients with liver caloric burden NASH Reversing fibrosis Dyslipidemia drives 30 Million is key to avoiding cardiovascular disease, the #1 US patients with NASH transplant, cancer, death cause of mortality by 2030 ©2022 AKERO THERAPEUTICS. 5

EFX Engineering Potentially Optimal for NASH Efficacy, With Convenient Once-weekly Dosing Key attributes Akero proprietary Increases half-life High affinity for Fc-FGF21, from < 2 hours β-Klotho Point mutations to 3-4 days Better translation Balanced potency Inactive to human at FGFR1c, 2c, 3c at FGFR4 pharmacology Stanislaus, S et al. (2017) Endocrinology 158(5): 1314-27; Lee, S et al. (2018) Nature 553: 501-505; Kharitonenkov, A et al. (2007) Endocrinology 148(2)774-781 ©2022 AKERO THERAPEUTICS. 6

EFX Acts on Two Major Sources of Liver Fat With Potential for Optimal Reduction Sources of Fat Flowing into and Acting on both hepatic and peripheral sources of liver Through Liver for Patients with NASH fat is key to optimizing liver fat reduction Dietary Fat Source of Liver Fat FGF Receptor EFX Activity (10-20%) Adipose Tissue: Lipolysis Lipolysis FGFR1c Liver: ü (40-50%) De Novo FGFR1c Lipogenesis (30-40%) FGFR2c, 3c FGFR2c De Novo Lipogenesis ü FGFR3c ©2022 AKERO THERAPEUTICS. 7

EFX Direct And Indirect Anti-fibrotic Effects Pathogenesis of Fibrosis Hepatocyte Stress & Death Kupffer Cell Activation Myofibroblast Differentiation Fibrogenesis EFX Anti-Fibrotic Activity Bao, L et al. (2018) Br J Pharmacol 175:3379-3393; Fisher, FM et al. (2014) Gastroenterology 147:1073-1083.e6; Jimenez, V et al. (2018) EMBO Mol Med 10:e8791; Lee, JH et al. (2016) *Cited literature available on company website Am J Transl Res 8:4750-4763; Sanyal, A et al. (2018) Lancet 392:2705-2717; Le, CT et al. (2018) PLOS one 13:e0192146; Xu, P et al. (2016) Toxicol Appl Pharmacol 290:43-53; Yu, Y et al. (2016) Int Immunopharmacol 38:144-152 ©2022 AKERO THERAPEUTICS. 8

Phase 2a Trial (Balanced) Design (F1-F3) 16 WEEKS Primary Endpoint • Absolute Liver Fat Placebo (n=20) Key Secondary Endpoints • Relative Liver Fat 28mg EFX (n=20) • Response Rate • ALT 50mg EFX (n=20) Key Exploratory Efficacy Endpoints • 2-Point NAS Improvement • Fibrosis Improvement • NASH Resolution 70mg EFX (n=20) • Serum Pro-C3 Screening Week 6 Week 12 Post-Treatment Liver Biopsy Subjects achieving ≥30% relative reduction of hepatic fat at MRI-PDFF week 12 eligible for post-treatment biopsy; biopsy scoring based on NASH CRN ©2022 AKERO THERAPEUTICS. 9 Screening Biopsy-Confirmed NASH Randomization Safety Follow-Up Responder Paired Biopsies

Phase 2a Expansion Cohort C Trial Design (F4) BALANCED study included an expansion cohort, Cohort C, of patients with compensated cirrhosis (F4), Child-Pugh Class A 16 WEEKS Primary Endpoint Safety & tolerability Placebo (n=10) EFX 50 mg (n=20) * Liver Biopsy * Protocol amended to include voluntary end-of-treatment biopsies FibroScan Fibrosis Biomarkers (ELF, Pro-C3) ©2022 AKERO THERAPEUTICS. 10 Screening Randomization

Baseline Demographics: Main Study & Cohort C a b BALANCED Main Study Cohort C Placebo EFX 28mg EFX 50mg EFX 70mg Placebo EFX 50mg Parameter Mean (N=21) (N=19) (N=20) (N=20) (N=10) (N=20) Age (Years) 52 50 53 53 57.1 61.1 Sex (Male/Female) 6/15 9/10 10/10 9/11 7/3 4/16 Weight (kg) 99.6 108.2 103.6 103.1 119.1 97.9 c c NAFLD Activity Score (NAS) 5.1 5.6 5.1 5.6 3.4 4.2 (range) (4 to 7) (4 to 7) (3 to 7) (5 to 7) (1 to 6) (1 to 7) Alanine Aminotransferase (ALT) (U/L) 50.7 62.5 53.4 56.8 32.7 31.7 Aspartate Aminotransferase (AST) (U/L) 38.6 41.1 35.4 44.6 28.9 31.4 % Type 2 Diabetes 67 37 50 50 50 50 HbA1c (%) 6.5 6.2 6.4 6.2 6.5 6.1 Triglycerides (mg/dL) 208 176 177 180 122 135 ELF Score 9.4 9.5 9.5 9.6 9.7 10.4 Pro-C3 (μg/L) 16.1 19.2 16.2 17.2 22.6 25.6 Liver Stiffness (kPA) 11.9 12.5 11.3 12.4 25.8 22.1 a b Full Analysis Set, F1-F3 (all subjects randomized into the BALANCED main study); Full Analysis Set, F4 (all subjects randomized into BALANCED Cohort C [except where otherwise c noted]); Liver Biopsy Analysis Set, F4 (all Cohort C subjects confirmed by central reader as F4 at baseline with Week 16 liver biopsy results) ©2022 AKERO THERAPEUTICS. 11

Substantial Reductions in Liver Fat at Week 12 Across All Dose Groups (F1-F3 NASH) Proportion of Patients Achieving Fat Reduction Thresholds LS Mean Reduction in Liver Fat Placebo 28mg 50mg 70mg Placebo 28mg 50mg 70mg Endpoint Placebo 28mg 50mg 70mg (N=20) (N=16) (N=17) (N=15) 0 -0% Relative Reduction in Liver Fat -3 ** *** *** ≥30% 10% 100% 100% 100% -6 ** *** *** ≥50% 5% 69% 100% 93% -9 * ** *** ≥70% 5% 50% 53% 80% -63% -71% -12 -72% *** Normalization of Liver -12.3 Fat Content *** -13.4 *** -14.1 -15 * ** *** ≤5% 5% 25% 53% 67% * ** *** *** p<0.001, versus placebo (ANCOVA) p<0.05, p<0.01, p<0.001, versus placebo (ANCOVA) Source Data: Full Analysis Set, F1-F3 ©2022 AKERO THERAPEUTICS. 12 Absolute Reduction (%)

High Rates of Fibrosis Improvement After 16 Weeks Across All Dose Groups (F1-F3 NASH) Fibrosis Improvement ≥1 Stage and 1,2 No Worsening of NASH 70% 70% 62% Biopsy Reading 60% 60% • All baseline and end-of-treatment 48% 50% 50% 46% biopsies were centrally read by a single NASH-CRN pathologist 40% 40% 36% • Baseline biopsies were not re-read with 30% 30% end-of-treatment biopsies 19 6 8 5 20% 20% • All biopsies were read blinded to both treatment assignment and patient 10% 10% 0% 0% 0% Overall Overall Placebo All EFX 28mg 50mg 70mg N=2 N=40 N=13 N=13 N=14 1 Improvement in liver fibrosis greater than or equal to one stage and no worsening of NASH (defined as no increase in NAS for ballooning, inflammation, or steatosis) 2 Secondary and exploratory histological endpoints were not powered for statistical significance Source Data: Liver Biopsy Evaluable Analysis Set, F1-F3 (all BALANCED main study responders who had baseline and end-of-treatment liver biopsy results) ©2022 AKERO THERAPEUTICS. 13

EFX F1-F3 Fibrosis Improvement in Context 1 Proportion of Patients with ≥1 Stage Improvement in Fibrosis and No Worsening of NASH Fc-FGF21 GlycoPEG-FGF21 PPAR Thyroid β GLP-1 Madrigal Akero Inventiva Madrigal Novo Nordisk Efruxifermin Pegozafermin Lanifibranor Resmetirom Semaglutide Weekly Injection Weekly Injection Daily Oral Daily Oral Daily Injection 16 Wks 20 Wks 24 Wks 36 Wks 72 Wks Increasing dosing 62% duration 48% 46% 46% 36% 34% 32% 29% 29% 26% 24% No Placebo Arm 0% Pbo 28mg 50mg Pbo 0.8g 1.2g Pbo All Pbo 0.2mg 0.4mg 27mg (N=19) (N=2) (N=13) (N=13) (N=62) (N=63) (N=69) (N=34) (N=79) (N=80) (N=78) (N=82) Note: These data are derived from different Phase 2 clinical trials at different points in time, with 89Bio (2022) January 24 Corporate Presentation; Inventiva (2020) June 16 Corporate Presentation; Harrison, S et al. (2019) Lancet differences in trial design and patient populations. No head-to-head clinical trials have been conducted. 394(10213):2012-24; Novo Nordisk (2020) June 19 R&D Investor Presentation. All trademarks are the property of their respective owners. 1 FDA Guidance for Industry: Noncirrhotic Nonalcoholic Steatohepatitis With Liver Fibrosis: Developing Drugs for Treatment (2018) ©2022 AKERO THERAPEUTICS. 14

Half of F2-F3 EFX Patients Achieved 2-Stage Fibrosis Improvement Fibrosis Change in EFX-Treated Patients Fibrosis Change in EFX-Treated Patients with Baseline F1-F3 Fibrosis (n=40) with Baseline F2 or F3 Fibrosis (n=22) 2-Stage 28% Improvement (n=11) 2-Stage 50% 55% Improvement (n=11) 68% 1-Stage 28% Improvement (n=11) 1-Stage 18% Improvement (n=4) 35% No Change (n=14) 23% No Change (n=5) Worsened 10% (n=4) Worsened 9% (n=2) 1 1 Source Data: Liver Biopsy Evaluable Analysis Set, F1-F3 ©2022 AKERO THERAPEUTICS. 15

Rapid Improvements in Fibrosis Biomarkers Consistent with Histological Improvements (F1-F3 NASH) Pro-C3, LS Mean (ug/L) Pro-C3, LS Mean Change from Baseline Dose Group Baseline Δ Week 12 BL W1 W4 W8 W12 Placebo 16.1 -1.5 10% *** 19.2 -6.1 28mg *** 16.2 -5.9 0% 50mg *** 70mg 17.2 -6.7 -10% -20% Enhanced Liver Fibrosis (ELF) Score, LS Mean -30% Dose Group Baseline Δ Week 12 *** *** *** -40% 9.4 0.0 Placebo *** *** *** *** *** *** 28mg 9.5 -0.7 *** -50% *** 9.5 -0.8 50mg Placebo 28mg 50mg 70mg * 9.6 -0.4 70mg *** * *** p<0.001, versus placebo (MMRM) p<0.05, p<0.001 versus placebo (ANCOVA) Source Data: Full Analysis Set, F1-F3 ©2022 AKERO THERAPEUTICS. 16
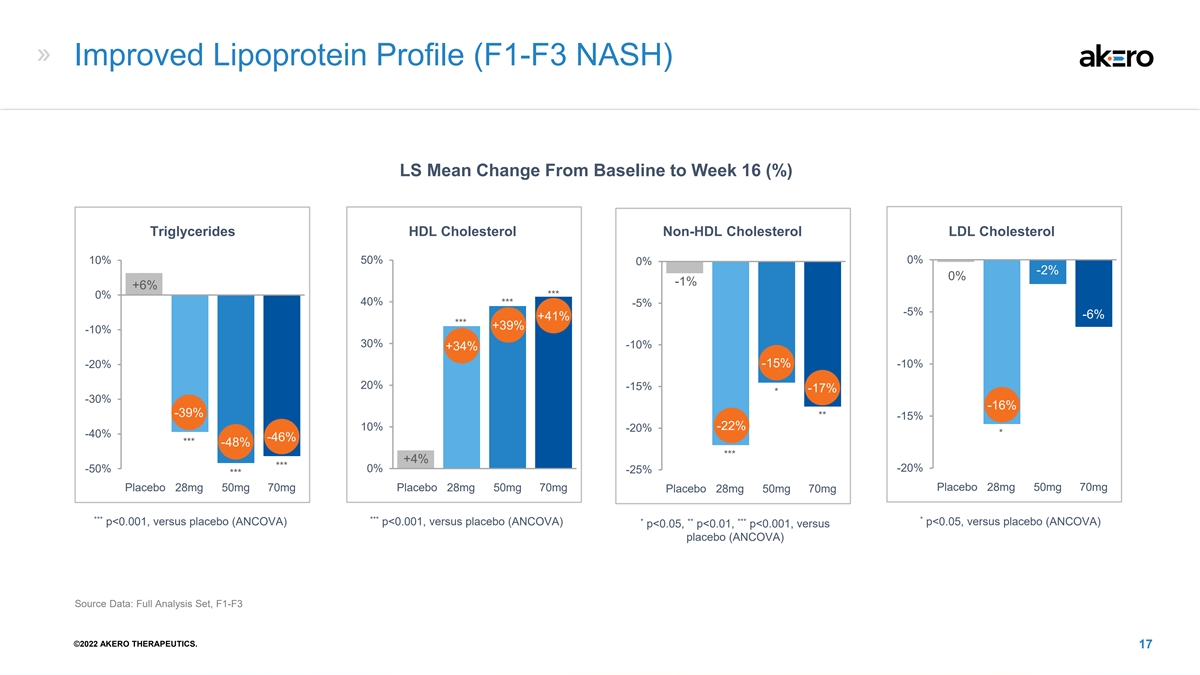
Improved Lipoprotein Profile (F1-F3 NASH) LS Mean Change From Baseline to Week 16 (%) Triglycerides HDL Cholesterol Non-HDL Cholesterol LDL Cholesterol 10% 50% 0% 0% -2% 0% -1% +6% *** 0% 40% *** -5% -5% -6% +41% *** +39% -10% 30% -10% +34% -15% -10% -20% 20% -15% -17% * -30% -16% -39% ** -15% -22% 10% -20% * -40% -46% *** -48% *** +4% *** -50% 0% -20% -25% *** W16 W16 W16 W16 Placebo 28mg 50mg 70mg Placebo 28mg 50mg 70mg Placebo 28mg 50mg 70mg Placebo 28mg 50mg 70mg *** *** * * ** *** p<0.001, versus placebo (ANCOVA) p<0.001, versus placebo (ANCOVA) p<0.05, versus placebo (ANCOVA) p<0.05, p<0.01, p<0.001, versus placebo (ANCOVA) Source Data: Full Analysis Set, F1-F3 ©2022 AKERO THERAPEUTICS. 17

High Response Rates on NASH Resolution After 16 Weeks Across All Dose Groups (F1-F3 NASH) 1,2 NASH Resolution and No Worsening of Fibrosis 70% 70% 60% 60% 54% 50% 48% 50% 50% 46% 43% 40% 40% 30% 30% * 1 19 6 7 6 20% 20% 10% 10% 0% 0% Overall Overall Placebo All EFX 28mg 50mg 70mg N=2 N=40 N=13 N=13 N=14 1 2 NAS score of 0 or 1 for lobular inflammation and a score of 0 for ballooning; Secondary and exploratory histological endpoints were not * powered for statistical significance; A single placebo responder lost 25 pounds over 16 weeks (11% weight reduction) Source Data: Liver Biopsy Evaluable Analysis Set, F1-F3 ©2022 AKERO THERAPEUTICS. 18

Substantial Reductions in Markers of Liver Injury After 16 Weeks of Treatment (F1-F3 NASH) ALT, LS Mean Change from Baseline AST, LS Mean Change from Baseline BL W1 W4 W8 W12 W16 W20 BL W1 W4 W8 W12 W16 W20 40% 40% 20% 20% 0% 0% *** -20% -20% ** *** ** *** *** *** *** *** *** *** *** *** *** *** *** *** *** -40% -40% *** *** *** *** *** *** -60% -60% Placebo 28mg 50mg 70mg Placebo 28 mg 50 mg 70 mg ** *** *** p<0.01, p<0.001, versus placebo (MMRM) p<0.001, versus placebo (MMRM) Similar dose-related improvements observed for GGT & ALP Source Data: Full Analysis Set, F1-F3 ©2022 AKERO THERAPEUTICS. 19

Clinically Meaningful Improvements in Glycemic Control After 16 Weeks (F1-F3 NASH) LS Mean Change From Baseline to Week 16 (%) 1 2 HbA1c C-Peptide All Patients (N=80) Patients with Type 2 DM (N=41) All Patients (N=80) Patients Type 2 DM (N=41) 0.1 0.2 20% 20% +21% +0.1 0.1 0.0 +0.0 -0.1 +0.1 0.0 -0.1 10% 10% -0.1 -0.2 -0.2 -0.3 0% 0% -0.4 -0.3 -5% -0.5 -0.4 -0.4 * -0.5 -0.6 -10% -10% ** -0.5 -0.6 -0.6 -22% -24% -0.7 -20% -20% -0.7 -27% -28% -0.9 * -29% -0.8 -0.8 -33% * -0.9 -0.9 -30% -30% * ** * * W16 W16 W16 W16 Placebo 28mg 50mg 70mg Placebo 28mg 50mg 70mg Placebo 28mg 50mg 70mg Placebo 28mg 50mg 70mg 1 2 Absolute change from baseline, % Relative percent change from baseline * ** * p<0.05, p<0.01, versus placebo (ANCOVA) p<0.05, versus placebo (ANCOVA) Source Data: Full Analysis Set, F1-F3 ©2022 AKERO THERAPEUTICS. 20

Weight Loss Observed For All Dose Groups (F1-F3 NASH) Mean Change in Body Weight (kg) Mean Change in Body Weight at Week 16 (kg) BL W1 W4 W8 W12 W16 0 +0.1 0 -0.3 -1 -1 -2 -2.3 -2 -3 -3 -3.7 -4 -4 W16 Placebo 28mg 50mg 70mg Placebo 28mg 50mg 70mg Source Data: Full Analysis Set, F1-F3 ©2022 AKERO THERAPEUTICS. 21

Consistent Results Observed for Relevant Endpoints (F1-F3) b b a Fibrosis Change in Patients HbA1c, % C-Peptide (%) Liver Fat (%) d,e,f with F2 or F3 at Baseline Placebo EFX 28mg EFX 50mg Placebo EFX 28mg EFX 50mg Placebo EFX 28mg EFX 50mg Pbo 28mg 50mg Pbo 28mg 50mg Pbo 28mg 50mg 0% 2-Stage +0.1 50% Improvement +21% 68% -0.1 1-Stage 18% Improvement No Change 23% -63% -22% -24% -0.4 -71% Worsened 9% *** * * * *** 2 c b b b Body Weight (kg) Triglycerides (%) HDL Cholesterol (%) Non-HDL Cholesterol (%) ≥1 Stage Fibrosis Improvement d,e and No Worsening of NASH PlPb aceo bo EFX 28m 28mg g EFX 50m 50g mg Pla Pb ceo bo 2 28m 8mgg 5 50m 0mgg PlPb aceo bo 2 28m 8mgg 5 50m 0mgg PPb lace o bo 2 28m 8mgg 5 50m 0mg g 62% *** -1% +0.1 +6% *** +41% -0.3 46% +39% -15% * -39% 0% -16% *** -2.3 -48% +4% Placebo EFX 28mg EFX 50mg *** Pbo 28mg 50mg *** a b c LS Mean Change from Baseline to Week 12; LS Mean Change from Baseline to Week 16; Mean Change from Baseline to Week 16; d e f Proportion of subjects; not powered for statistical significance; Includes all EFX-treated patients, including 70mg dose * p<0.05, *** p<0.001, versus placebo (ANCOVA) ©2022 AKERO THERAPEUTICS. 22

Drug-related Treatment-Emergent Adverse Events (TEAE) (F1-F3) Most Frequent (>10%) Placebo All EFX EFX 28mg EFX 50mg EFX 70mg * (N=21) (N=58) (N=19) (N=19) (N=20) Drug-Related AEs Diarrhea 2 (10%) 21 (36%) 5 (26%) 10 (53%) 6 (30%) Nausea 0 (0%) 20 (34%) 6 (32%) 4 (21%) 9 (45%) Increased appetite 1 (5%) 13 (22%) 4 (21%) 4 (21%) 5 (25%) Vomiting 0 (0%) 9 (16%) 5 (26%) 2 (11%) 2 (10%) Frequent bowel movements 0 (0%) 8 (14%) 3 (16%) 2 (11%) 3 (15%) Abdominal pain 0 (0%) 7 (12%) 1 (5%) 3 (16%) 3 (15%) Injection site erythema 0 (0%) 7 (12%) 2 (11%) 0 (0%) 5 (25%) Injection site reaction 0 (0%) 6 (10%) 2 (11%) 1 (5%) 3 (15%) Fatigue 2 (10%) 6 (10%) 0 (0%) 1 (5%) 5 (25%) TEAE/SAE Disposition Placebo All EFX 28mg 50mg 70mg TEAE Leading to Death 0 0 0 0 0 a b c TEAE Leading to Discontinuation 1 6 2 0 4 d Serious Adverse Event (SAE) 0 2 1 0 1 * Across EFX dose groups a b Muscular Weakness & Myalgia; Nausea, Vomiting & Dysgeusia; Panic Attack and Anxiety-Linked Tremor; c d Dysphagia (Not Drug Related); Acute Pancreatitis (also an SAE); Vomiting; Fatigue & Nausea; Related to pre-dosing liver biopsy Source Data: Safety Set, F1-F3 (all BALANCED main study subjects who received at least one dose of study drug) ©2022 AKERO THERAPEUTICS. 23

Value of Preventing Progression to & Reversing From Cirrhosis Survival Free of Liver Transplantation 100% 80% F0 ~50% 60% 5-year mortality 50% F1 without liver transplant 40% F2 for F4 patients F3 20% F4 P<0.001 0% Follow-up (years) Angulo, P et al. (2015) Gastroenterology 149:389-397 ©2022 AKERO THERAPEUTICS. 24 Cumulative Survival (%)

Cirrhosis Regression is Associated with Improved Clinical Outcomes Pooled treatment groups from STELLAR 4 and simtuzumab cirrhosis study Hazard Ratio: 0.16 Hazard Ratio: 0.08 p=0.0104 p=0.0004 9 8.3 8 7.2 7 No fibrosis regression 6 Fibrosis regression 5 4 3 2 1.1 0.7 1 2/176 69/957 69/834 2/300 0 NASH CRN fibrosis stage Ishak fibrosis stage Cirrhosis regression observed in 16% of patients (treatment and placebo groups) over 48 weeks Note: These data are derived from different clinical trials at different points in time, with differences in trial design and patient populations. No head-to-head clinical trials have been conducted. Sanyal A, et al. AASLD TLMdX2020. #90 ©2022 AKERO THERAPEUTICS. 25 Liver-related events (%)

Fibrosis Improvement Observed in Patients with Pre-cirrhotic (F1-F3) and Compensated Cirrhotic (F4) NASH Fibrosis Improvement 1,2 Improvement ≥1 Stage and No Worsening of NASH F1-F3 F4 70% 70% 60% 60% 62% 50% 50% 40% 40% 30% 30% 33% 20% 20% 10% 10% 0% 0% 0% 0% Placebo EFX 50mg Pb Plac oebo EF 5 X 5 0m 0g mg Pbo 50mg 1 Improvement in liver fibrosis greater than or equal to one stage and no worsening of NASH (defined as no increase in NAS for ballooning, inflammation, or steatosis) 2 Secondary and exploratory histological endpoints were not powered for statistical significance Source Data: Liver Biopsy Analysis Set, F4; Liver Biopsy Analysis Set, F4 ©2022 AKERO THERAPEUTICS. 26

EFX F4 Fibrosis Improvement in Context FGF21 ASK-1 ACC FXR GAL-3 LOXL2 Belapectin Efruxifermin Selonsertib Firsocostat Cilofexor Simtuzumab 40 48 Wks 48 Wks 48 Wks 52 Wks 96 Wks Increasing 16 Wks dosing 33% duration 30 20 16% 15% 14% 14% 14% 13% 13% 13% 10 8% 8% 7% 5% 0% 2% 0 Pbo EFX 50mg Pbo 6mg 18mg Pbo 20mg Pbo 30mg Pbo 2mpk 8mpk Pbo 200mg 700mg (n=5) (n=12) (n=172) (n=351) (n=354) (n=21) (n=20) (n=21) (n=19) (n=45) (n=46) (n=41) (n=75) (n=75) (n=79) * Results from all publicly reported NASH Phase 2 clinical trials in patients with compensated cirrhosis due to NASH reporting either ≥ 1-stage Note: These data are derived from different clinical trials at different points in time, fibrosis improvement (belapectin and simtuzumab) or ≥ 1-stage fibrosis improvement and no worsening of NASH (selonsertib, firsocostat and with differences in trial design and patient populations. No head-to-head clinical cilofexor); numerical values represent percent responders trials have been conducted. ©2022 AKERO THERAPEUTICS. 27
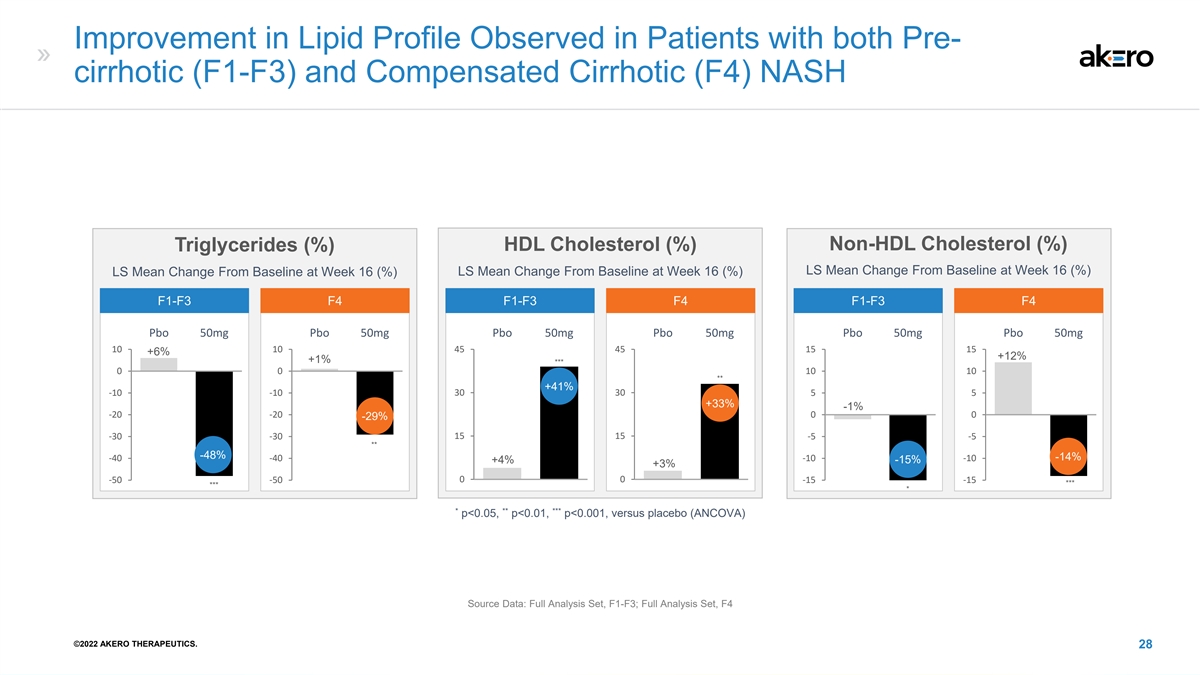
Improvement in Lipid Profile Observed in Patients with both Pre- cirrhotic (F1-F3) and Compensated Cirrhotic (F4) NASH Non-HDL Cholesterol (%) Triglycerides (%) HDL Cholesterol (%) LS Mean Change From Baseline at Week 16 (%) LS Mean Change From Baseline at Week 16 (%) LS Mean Change From Baseline at Week 16 (%) F1-F3 F4 F1-F3 F4 F1-F3 F4 Pbo 50mg Pbo 50mg Pbo 50mg Pbo 50mg Pbo 50mg Pbo 50mg 10 10 45 45 15 15 +6% +12% +1% *** 0 0 10 10 ** +41% -10 -10 30 30 5 5 +33% -1% -20 -20 0 0 -29% 15 15 -30 -30 -5 -5 ** -48% -40 -40 -10 -10 -14% +4% -15% +3% 0 0 -50 -50 -15 -15 *** *** * * ** *** p<0.05, p<0.01, p<0.001, versus placebo (ANCOVA) Source Data: Full Analysis Set, F1-F3; Full Analysis Set, F4 ©2022 AKERO THERAPEUTICS. 28

Improved Glycemic Control Observed in Patients with both Pre- cirrhotic (F1-F3) and Compensated Cirrhotic (F4) NASH HbA1c, % C-peptide (%) LS Mean Change From Baseline at Week 16 (%) LS Mean Change From Baseline at Week 16 (%) F1-F3 F4 F1-F3 F4 Placebo EFX 50mg Pbo 50mg Pbo 50mg Placebo EFX 50mg Placebo EFX 50mg Placebo EFX 50mg Pbo 50mg Pbo 50mg 0.1 0.1 24% 24% +0.1 +0.1 0.0 0.0 +21% 16% 16% -0.1 -0.1 8% 8% -0.2 -0.2 0% 0% -7% -0.3 -0.3 * * -0.4 -0.4 -8% -8% -0.4 -0.4 -20% -16% -16% * * -22% -0.5 -0.5 -24% -24% Absolute change from baseline, % Relative percent change from baseline * p<0.05, versus placebo (ANCOVA) Source Data: Full Analysis Set, F1-F3; Full Analysis Set, F4 ©2022 AKERO THERAPEUTICS. 29

Weight Loss Observed in Patients with both Pre-cirrhotic (F1-F3) and Compensated Cirrhotic (F4) NASH Body Weight LS Mean Change From Baseline at Week 16 (Kg) F1-F3 F4 1.5 1.5 1.0 1.0 +1.2 0.5 0.5 +0.5 0.0 0.0 -0.5 -0.5 -1.0 -1.0 -1.7 -1.5 -1.5 -2.2 -2.0 -2.0 -2.5 -2.5 Placebo EFX 50mg Placebo EFX 50mg Pbo 50mg Pbo 50mg Source Data: Full Analysis Set, F1-F3; Full Analysis Set, F4 ©2022 AKERO THERAPEUTICS. 30

Interpreting the Rapid Reversal of Fibrosis Observed in EFX-treated Patients with NASH ↓ Inflammation ↓ Liver fat ↓ Collagen Synthesis ↓ Hepatocyte Stress Directly anti-fibrotic Indirectly anti-fibrotic EFX • Fibrosis reversal in patients with compensated cirrhosis (F4), • Addressing underlying NASH disease drivers may indirectly two-stage improvement of fibrosis in patients with F2/F3 NASH, contribute to fibrosis reversal for patients with F1-F3 NASH with and corroborating non-invasive markers of fibrosis improvement adequate time for the liver to regenerate in only 16 weeks likely reflects direct anti-fibrotic activity • Redress of the underlying NASH disease drivers is necessary to • Fibrosis reversal is especially advantageous for patients with sustain fibrosis reversal across all fibrosis stages cirrhotic NASH who face high risk of mortality and severe • Supports broader metabolic improvements morbidity ©2022 AKERO THERAPEUTICS. 31

EFX Positioning as Potential Best-in-Class NASH Therapy Liver Fat Reduction Consistent Fibrosis Reversal Activity NASH Resolution Reduced Cardiovascular Risk EFX+ F2-F4 Restored Healthy Lipid Profile Enhanced Insulin Sensitivity Better Glycemic Control Monotherapy Weight Loss ©2022 AKERO THERAPEUTICS. 32

Building Foundation for Phase 3: Parallel Phase 2b Trials Phase 2a Phase 2b F1-F3 F4 F2/F3 F4 Biopsy-Confirmed: Cohort C BALANCED HARMONY SYMMETRY (Expansion of BALANCED) Enrollment complete; Status Completed Completed Readout expected Initiated Jul’21 in 3Q’22 Duration 16 Weeks 16 Weeks 24 Weeks 36 Weeks EFX Doses 28, 50, 70mg 50mg 28, 50mg 28, 50mg Placebo-Controlled üüüü ©2022 AKERO THERAPEUTICS. 33

HARMONY Trial Design: Non-Cirrhotic NASH (F2/F3) Key Inclusion Criteria Key Secondary Efficacy Endpoints Phase 2b Primary Endpoint • F2-3 NASH • Fibrosis Improvement • NASH Resolution • Weight Change • NAS ≥4 • Fibrosis Markers • MRI-PDFF • Liver fat ≥8% • Lipoproteins • Liver Injury Markers • Glycemic Control Long-Term Safety Follow-Up 24 Weeks Placebo Placebo EFX 28mg * EFX EFX 50mg Liver Biopsy EOP2 * Phase 3 Initiation MRI-PDFF Meeting * Phase 3 protocol finalization expected after end-of-Phase 2 meeting with FDA ©2022 AKERO THERAPEUTICS. 34 Phase 2b Screening Randomization

SYMMETRY Trial Design: Cirrhosis Due to NASH (F4) Key Inclusion Criteria Key Secondary Efficacy Endpoints Phase 2b Primary Endpoint • F4 NASH • Fibrosis Improvement • NASH Resolution • Glycemic Control (Cirrhosis reversal) • Fibrosis Markers • Weight Change • Lipoproteins • Liver Injury Markers Long-Term Safety Follow-Up 36 Weeks Placebo Placebo EFX 28mg * EFX EFX 50mg * Pending finalization of Phase 3 protocol Liver Biopsy ©2022 AKERO THERAPEUTICS. 35 Phase 2b Screening Randomization

No Paired Reads • Shuffled Slide Sequence Histopathology Scoring of Biopsies in HARMONY and SYMMETRY Key Features of EFX Biopsy Analysis Plan Biopsy Analysis Flow Chart • Incorporates FDA input on EFX Phase 2b trial designs, Slides Prepared From Tissue Samples reflecting efforts to increase liver biopsy reliability • All biopsies read by two, experienced pathologists well- versed in NASH-Clinical Research Network (CRN) scoring Score 1 Score 2 system • Pathologists undergo training to align on interpretation of histology Same Score Different Scores • Each screening and on-treatment biopsy scored in parallel by same two pathologists Consensus Meeting • Both blinded to subject ID and visit ID • Screening and end-of-treatment biopsies for a single Consensus Score No Consensus patient are not read simultaneously as paired samples • Randomized shuffling of screening biopsy slides and on- treatment biopsy slides to minimize temporal bias Adjudicated Score • Consensus meeting to resolve any scoring discrepancies Final Score QC’d and Reported to Study Portal • In absence of consensus, a third, equally qualified and trained pathologist adjudicates to finalize score Designed to Minimize Variability of Evaluation ©2022 AKERO THERAPEUTICS. 36 Blinded Subject ID • Blinded Timing ID

Evaluating EFX for Potential Use with GLP-1 Receptor Agonists Cohort D Design: Non-Cirrhotic NASH (F1-F3) 12 WEEKS Primary Endpoint • Safety & Tolerability GLP-1 Receptor Agonist + Placebo (n=10) Secondary Endpoints Key Inclusion Criteria • HbA1c • HbA1c ≥ 7.0% and ≤10.5% • Fibrosis Markers • Stable Dose of GLP-1 • MRI-PDFF • Insulin/ C-peptide GLP-1 Receptor Agonist + EFX 50 mg (n=20) • Lipoproteins • Weight Change • ALT/AST Liver Biopsy MRI-PDFF FGF21 and GLP-1 have complementary mechanisms of action, respectively as an insulin sensitizer and an insulin secretagogue ©2022 AKERO THERAPEUTICS. 37 Screening Randomization 30-day Follow-up

Supplying API and Drug Product/Device for Phase 3 Drug Substance (API) Drug Product/Device Combination Commercial scale Commercially precedented Released for Phase 3 1 mL SC weekly injection Comparability demonstrated Self-administered, stable at 2-8ºC ©2022 AKERO THERAPEUTICS. 38

Strong Financial Position 1 COMPLETED COMPLETED UPSIZED CASH UPSIZED IPO ON HAND FOLLOW-ON OFFERING June 20, 2019 As of March 31, 2022 June 10, 2020 ~$ ~$ 106M 216M ~$ 165M Raised in aggregate Raised in aggregate gross proceeds gross proceeds 1 Priced upsized IPO at top of Priced upsized follow-on offering at Cash sufficient to fund current operating marketing range top of marketing range plan into the third quarter of 2023 1 Cash, cash equivalents and short-term marketable securities ©2022 AKERO THERAPEUTICS. 39

Backup Slides

Patient Disposition (Balanced Main Study) Randomized (80) Randomized Not Dosed EFX (1) Dosed (79) Discontinued (11) Administrative Due to AEs EFX (4) EFX (6) - Pbo (1) Week 12 MRI-PDFF (68) Non-Responders Placebo (18) MRI-PDFF Responders (50) (≥ 30% Relative Reduction) Biopsies Not Collected Due to COVID-19 EFX (8) End-of-Treatment Biopsies Collected (42) Placebo (2) 28mg (13) 50mg (13) 70mg (14) ©2022 AKERO THERAPEUTICS. 41

Improvements in Fibrosis Biomarkers in Patients with Cirrhotic NASH Support Histology Results (F4) LS Mean Change From Baseline to Week 16 1 Liver Stiffness (kPa) Pro-C3 (µg/L) ELF Score 0 0 0.3 +0.3 0.2 -1 -2 -1.9 -3.4 0.1 -2 -4 0.0 -3 -0.1 -6 -4 -0.2 * -9.0 -8 †† -5 -5.7 -0.3 ** -0.4 -6 -10 -0.4 CFB CFB CFB Placebo EFX 50mg Placebo EFX 50mg Placebo EFX 50mg n=10 n=20 n=10 n=20 n=10 n=20 1 * ** Measured by FibroScan p<0.05, versus placebo (ANCOVA) p<0.01, versus placebo (ANCOVA) †† p<0.01, versus baseline (ANCOVA) Source Data: Biomarker Analysis Set, F4 (all Cohort C subjects with baseline and interpretable on-study measure of ELF or pro-C3, respectively); Liver Stiffness Analysis Set, F4 (all Cohort C subjects with baseline and interpretable on-study measure of Liver Stiffness) ©2022 AKERO THERAPEUTICS. 42

Tolerability Overview (F4) Most Frequent (>15%) Placebo EFX 50mg Drug-Related AEs (N=10) (N=17) Key Observations Diarrhea 1 (10%) 7 (41%) • Encouraging tolerability given population with more advanced Nausea 1 (10%) 5 (29%) disease Injection site reaction 0 5 (29%) • All injection site AEs Grade 1 Injection site erythema 0 4 (24%) • No reports of tremor Headache 0 3 (18%) TEAE/SAE Disposition Placebo EFX 50mg a b Study Discontinuations 1 1 c Serious Adverse Events (SAE) 1 0 Deaths 0 0 a Withdrawal of consent b abdominal distension, constipation, diarrhea, pruritus c pulmonary embolism Source Data: Safety Set, F4 (all Cohort C subjects confirmed by central reader as F4 at baseline who received at least one dose of study drug) ©2022 AKERO THERAPEUTICS. 43

NASH Resolution Also Observed in Patients with Cirrhotic NASH (F4) 1,2 NASH Resolution 30% Change in NAS among Patients Achieving NASH Resolution 25% EFX Subject Baseline NAS Week 16 NAS 20% A 7 1 B 3 1 C 6 1 3 10% Proportion of Patients with ≥2 Point NAS Reduction Placebo EFX 50mg 0% 0% 1 (20%) 7 (58%) Overall Placebo EFX 50mg n=5 n=12 1 NAS score of 0 or 1 for lobular inflammation and a score of 0 for ballooning 2 Study not powered to assess statistical significance of histological endpoints Source Data: Liver Biopsy Analysis Set, F4 ©2022 AKERO THERAPEUTICS. 44

AKERO THERAPEUTICS 601 Gateway Boulevard NASDAQ: AKRO Suite 350 South San Francisco, CA 94080
