UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): September 5, 2019
Akero Therapeutics, Inc.
(Exact name of registrant as specified in its charter)
|
Delaware |
|
001-38944 |
|
81-5266573 |
|
(State or other jurisdiction |
|
(Commission |
|
(I.R.S. Employer |
|
170 Harbor Way, 3rd Floor |
|
94080 |
|
(Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code (650) 487-6488
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Trading |
|
Name of each exchange on which registered |
|
Common Stock, par value $0.0001 per share |
|
AKRO |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Item 5.02. Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers.
To fill an existing vacancy on the Audit Committee of the Board of Directors (the “Audit Committee”) of Akero Therapeutics, Inc. (the “Company”), effective as of September 5, 2019, the Company’s Board of Directors appointed a current independent director, Dr. Kevin Bitterman, to serve as a member of the Audit Committee. The Board of Directors has determined that Dr. Bitterman meets the requirements for independence and financial literacy of audit committee members under the applicable listing standards of The Nasdaq Stock Market LLC and the Securities Exchange Act of 1934, as amended.
Item 7.01. Regulation FD Disclosure.
The Company from time to time presents and/or distributes to the investment community at various industry and other conferences slide presentations to provide updates and summaries of its business. A copy of its current corporate slide presentation is attached to this Current Report on Form 8-K as Exhibit 99.1. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.1.
The information contained in Item 7.01 in this Current Report on Form 8-K (including Exhibit 99.1) shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934 (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933 or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
|
Exhibit |
|
Description |
|
|
|
|
|
99.1 |
|
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
| |
|
Date: September 6, 2019 |
AKERO THERAPEUTICS, INC. | |
|
|
|
|
|
|
By: |
/s/ Andrew Cheng |
|
|
|
Andrew Cheng, M.D., Ph.D. President and Chief Executive Officer |
RESTORING METABOLIC BALANCE. TRANSFORMING LIVES. CORPORATE PRESENTATION Developing medicines to reverse the course of serious metabolic diseases.

Safe Harbor This presentation has been prepared by Akero Therapeutics, Inc. (“we,” “us,” “our,” “Akero” or the “Company”) and is made for informational purposes only and does not constitute an offer to sell or a solicitation of an offer to buy securities, nor shall there be any sale of any securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. The information set forth herein does not purport to be complete or to contain all of the information you may desire. Statements contained herein are made as of the date of this presentation unless stated otherwise, and neither this presentation, nor any sale of securities, shall under any circumstances create an implication that the information contained herein is correct as of any time after such date or that information will be updated or revised to reflect information that subsequently becomes available or changes occurring after the date hereof. This presentation may contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to our business, operations, and financial conditions, including but not limited to current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our development plans, our preclinical and clinical results and other future conditions. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward- looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source. 2

AKERO Building Value on a Strong Foundation 3 AKR-001, Fc-FGF21 fusion protein, licensed from Amgen 3-4 day half-life for QW dosing Robust effects on lipoproteins and markers of insulin sensitivity in 83 T2D patients in two Phase 1 trials Well tolerated Currently in Phase 2a FGF analogs observed to reduce liver fat and reverse fibrosis in NASH patients AKR-001 expected to (a) have metabolic & anti-fibrotic effects and (b) target both liver & adipose tissue With balanced receptor activity, AKR-001 has potential to be leading FGF analog 1Q20 Ph2a clinical trial primary assessment (MRI-PDFF) 2Q20 Ph2a clinical trial repeat liver biopsy assessments Clinical-Stage FGF Analog Precedented Path in NASH Key Upcoming Milestones

4 LEADERSHIP Andrew Cheng, MD, PhD President & CEO 19 years at Gilead Chief Medical Officer & HIV Division Head Major role in 11 NDA/MAA approvals Jonathan Young, PhD, JD Founder, EVP & COO Over 15 years in biotechnology product development, law and regulatory policy General Counsel and VP Policy, Braeburn Partner and General Counsel, FoxKiser William White EVP, CFO & Head of Corporate Development 18 years in life sciences investment banking at Goldman Sachs, Citigroup and Deutsche Bank Most recently, head of US Life Sciences Investment Banking at Deutsche Bank Advised on more than $70bn in M&A and $25bn in financing transactions Confidential and Proprietary Tim Rolph, D.Phil Founder & CSO Over 30 years at Pfizer & Glaxo CSO of Pfizer’s cardiovascular and metabolic disease unit Head of Groton & UK Discovery Research, Pfizer Major role in discovery and early clinical evaluation of two medicines: Selzentry (HIV) and Steglatro (Diabetes) Kitty Yale EVP & Chief Development Officer Over 25 years Gilead, Roche, Pfizer VP Gilead Worldwide Clinical Operations Major role in 8 global approvals NDA, MAA , JNDA and CFDA EXTENSIVE EXPERIENCE IN Drug discovery Development Commercialization INVOLVED IN 20+ MEDICINE APPROVALS

5 COMPLETED UPSIZED IPO June 20, 2019 $16 Price upsized IPO at top of marketing range ~$106M Raised in aggregate gross proceeds Q2 EARNINGS UPDATE June 30, 2019 $7.6M Q2 operating expenses ~$163M Cash and cash equivalents FUNDED THROUGH 2022 Phase 2b STRONG FINANCIAL POSITION Projected completion by EOY 2022 Phase 2a To be completed in 1H 2020

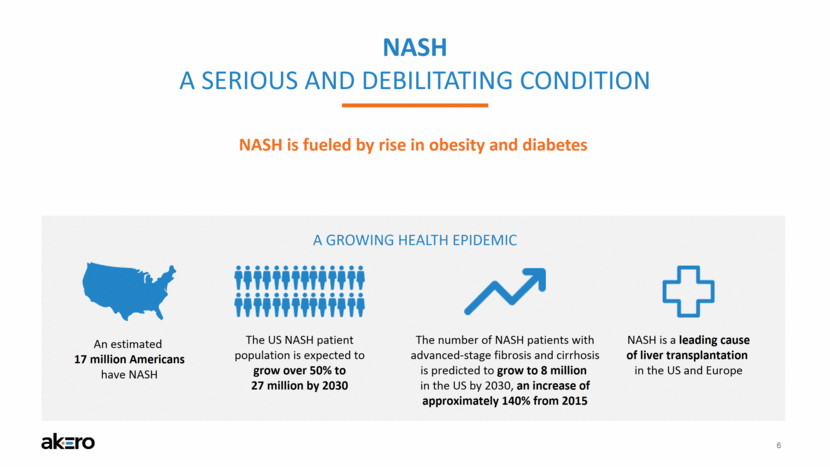
6 NASH A SERIOUS AND DEBILITATING CONDITION An estimated 17 million Americans have NASH The US NASH patient population is expected to grow over 50% to 27 million by 2030 A GROWING HEALTH EPIDEMIC The number of NASH patients with advanced-stage fibrosis and cirrhosis is predicted to grow to 8 million in the US by 2030, an increase of approximately 140% from 2015 NASH is a leading cause of liver transplantation in the US and Europe NASH is fueled by rise in obesity and diabetes
7 FGF21 TARGETS ALL CORE STAGES OF NASH PATHOGENESIS Excessive Caloric Burdening of Liver Excessive Liver Fat, Fat Oxidation Hepatocyte cell stress, injury, death Inflammation Fibrosis Improve insulin sensitivity Redirect calories away from liver without weight gain Reduce all three sources of liver fat Reduce de novo lipogenesis Reduce fat oxidation Improve lipoproteins Reduce oxidative stress & lipotoxicity Reduce ER stress and cumulative mitochondrial damage Suppress cell death Suppress activation of pro-inflammatory Kupffer cells Suppress activation of collagen-producing hepatic stellate cells DISEASE BURDEN NASH PATHOGENESIS FGF21 INTERVENTION

UNDERSTANDING THE SOURCES OF LIVER FAT AND RELEVANT FGF RECEPTORS 8 Source of Liver Fat FGF Receptor FGF21 Activity Lipolysis FGFR1c De Novo Lipogenesis FGFR2c FGFR3c Lambert, JE et al. (2014) Gastroenterology 146(3): 726-35 Acting on both hepatic and peripheral sources of liver fat is key to optimizing liver fat reduction Sources of Fat Flowing Into and Through Liver for NASH Patients Adipose Tissue: Lipolysis (40 - 50%) FGFR1c Liver: De novo Lipogenesis (30 - 40%) FGFR2c, 3c Dietary Fat (10 - 20%)

Impact of Balanced fgfr Agonism on Fat Reduction Liver fat reduction of 67% (MRI-PDFF) largest reported among all candidates that we know to be targeting FGFRs Balanced potency at FGFR1c, 2c, 3c appears to drive liver fat reduction FGFR4 appears unnecessary for glucose sensitivity & liver fat reduction 9 Not placebo-corrected -67% Hepatic (2c, 3c, 4) Adipose (1c) + Note: This data is derived from different clinical trials at different points in time, with differences in trial design and patient populations. No head-to-head clinical trials have been conducted Sanyal, A et al. (2018) Lancet 392(10165): 2705-17 ; Shankar, S et al. (2018) AASLD Poster; Harrison, S et al. (2018) EASL Presentation; Ge, H et al. (2014) J Biol Chem 289(44): 30470-80; Yu, X et al. (2013) PLOS ONE 8(7): e66923; Wu, X et al. (2013) J Lipid Res 54(2): 325-32; Wu, A-L et al. (2013) PLOS ONE 6(3): e17868; Wu, X et al. (2009) Proc Natl Acad Sci 106(34): 14379-84; Huang, X et al. (2007) Diabetes 56(10): 2501-10 Aldalfermin FGF19 (3mg, QD) - 26% - 38% - 37% - 0.67 -80% -60% -40% -20% 0% pegbelfermin PEG-FGF21 (20mg, QW) pegbelfermin PEG-FGF21 (10mg, QD) MRK-3655 FGFR1c/B-Klotho (240mg, 1 dose) NGM282 FGF19 (3mg, QD) Relative Fat Reduction (MRI - PDFF) Adipose (1c) Hepatic (2c, 3c) + Hepatic 2c, 3c, 4 Adipose 1c

Potential for Improved Liver Histology Within 12 Weeks aldafermin (NGM282) appeared to improve biopsy-based measures in NASH patients after 12 weeks Underscores the capacity of -Klotho-dependent FGFR agonism to improve NASH and reverse fibrosis May open up opportunity to accelerate path to registrational trials, via early demonstration of histological improvement Underscores potential for AKR-001 Balanced agonism of FGR1c, 2c and 3c Reduction in hepatocyte stress and apoptosis Direct and indirect anti-fibrotic effects 10 Mean histology change from baseline at W12 (% of patients) aldafermin 3 mg (n=19) Fibrosis 1 stage reduction 42% NAS 1 point reduction 84% Steatosis 1 point improvement 74% Lobular inflammation 1 point improvement 42% Hepatocellular Ballooning 1 point improvement 53% Shankar, S et al. (2018) AASLD Poster

AKR-001 Engineering Potentially Optimal for NASH Efficacy Key attributes 11 Stanislaus , S et al. (2017) Endocrinology 158(5): 1314-27; Lee, S et al. (2018) Nature 553: 501-505; Kharitonenkov, A et al. (2007) Endocrinology 148(2)774-781 Amgen-designed Fc-FGF21, Point mutations Increases half-life from < 2 hours to 3-4 days High affinity for -Klotho Balanced potency at FGFR1c, 2c, 3c Better translation to human pharmacology Inactive at FGFR4

AKR-001 Reproduced Balanced In Vitro Potency 12 Pharmacology of other FGF21 analogs has not been disclosed for individual receptors AKR-001, rhFGF21, and rhFGF19 Activation of FGFR-Dependent Intracellular Signaling in L6 Cells Stanislaus, S et al. (2017) Endocrinology 158(5):1314-1327

Improved Lipoproteins Weight Neutral 13 PROMISING DATA FROM PHASE 1 CLINICAL TRIALS And support AKR-001’s potential, in NASH patients, to be METABOLIC Redirect calories away from the liver Reduce liver fat Reduce hepatocellular stress and cell death ANTI-FIBROTIC Suppress inflammation Reverse fibrosis Improved Insulin Sensitivity Two Phase 1 clinical trials of AKR-001 studying 83 patients with type 2 diabetes showed

14 Percent Change From Baseline, Placebo, 21 mg QW and 70 mg QW a Day 25; b Day 29 ** = p<0.01; *** = p<0.001 AKR-001’s significant improvements in lipoproteins and reduction in adipose lipolysis are consistent with effective agonism of FGFR2c and 3c in the liver and FGFR1c in adipose tissue AKR-001 IMPROVED LIPOPROTEIN PROFILE *** -66% Triglyceridesa -25% LDL-Ca +51% HDL-Ca -36% ** Non-HDL-Ca *** ** -51% ApoBb

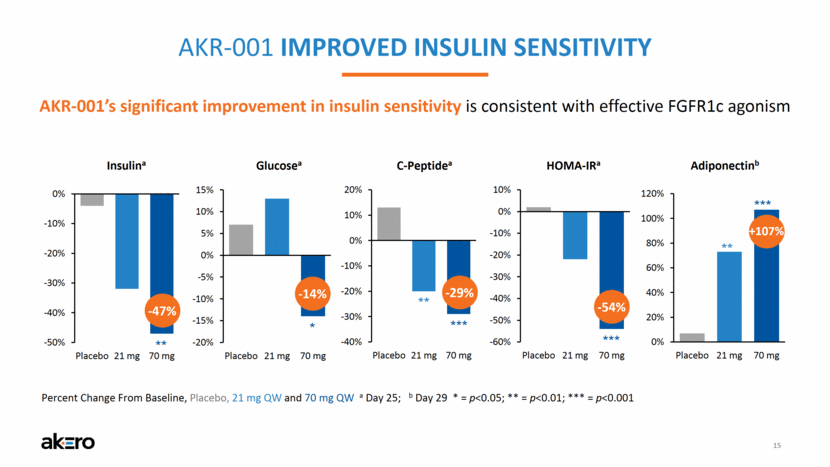
AKR-001’s significant improvement in insulin sensitivity is consistent with effective FGFR1c agonism Percent Change From Baseline, Placebo, 21 mg QW and 70 mg QW a Day 25; b Day 29 * = p<0.05; ** = p<0.01; *** = p<0.001 15 AKR-001 IMPROVED INSULIN SENSITIVITY -29% -54% +107% -14% -47% Insulina Glucosea C-Peptidea HOMA-IRa Adiponectinb -50% -40% -30% -20% -10% 0% Placebo 21 mg 70 mg **
AKR-001 Improved Lipoproteins Rapidly and Durably 16 AKR-001 70 mg QW effects on lipoproteins in Phase 1b clinical trial -8,200 -5,700 -3,200 -700 1,800 4,300 6,800 -70% -50% -30% -10% 10% 30% 50% 1 7 14 21 28 35 42 49 56 Serum AKR - 001 (ng/mL) Change from Baseline Study Day HDL-C LDL-C Triglycerides Serum AKR-001 *** *** *** *** *** *** *** *** *** *** *** *** *** ** * *** *** *** *** *** *** *** *** * ** * ** *** *** ** * 2 nd Dose 3 rd Dose 4th Dose * = p <0.05 ** = p <0.01 *** = p <0.001

17 PHASE 2A TRIAL: THE BALANCED STUDY NUMBER OF SUBJECTS About 80 PRIMARY ENDPOINT Absolute change from baseline in hepatic fat fraction (MRI-PDFF) at week 12 A Phase 2a multicenter, randomized, double-blind, placebo-controlled, dose-ranging trial to evaluate the safety, tolerability and efficacy of AKR-001 in biopsy-confirmed patients with NASH MRI-PDFF Liver Biopsy 6 WEEKS 16 WEEKS 4-8 WEEKS Screening Randomization AKR-001 70 mg (n=20) QW SC Injection AKR-001 50 mg (n=20) QW SC Injection AKR-001 28 mg (n=20) QW SC Injection Placebo (n=20) QW SC Injection Liver Biopsy Safety FU – Week 20 Week 6 Week 12 Screening Weeks 20-24

Milestones DELIVERED Milestones PROJECTED JUN 8 Q1 Q2 $45.5M Series B MRI-PDFF Read-out May 24 MAR 13 JUN 20 JUL 2 1st Patient Dosed IND Active ~$106M Upsized IPO Amgen License $45M Series A DEC 5 Liver Biopsy Read-out 2018 2019 2020 18

THANK YOU. CORPORATE PRESENTATION

